2010 NORTHEASTERN NATURALIST 17(Monograph 7):1–32
Invasive Shrubs in Kentucky
Richard L. Boyce*
Abstract - I surveyed the distribution of invasive (non-native) shrubs in Kentucky,
along with their properties, effects, and control measures. Kentucky's floral, physical
geography, and climate are representative of a much broader region of the US, and
the state has a large number of counties with available data. Invasive shrub species
richness increases with county population and appears to be underreported in most of
the state. The most widespread of the 68 species reported were Rosa multiflora (Multiflora Rose), Elaeagnus umbellate (Autumn Olive), Morus alba (Mulberry), Lonicera
maackii (Amur Honeysuckle), Albizia julibrissin (Mimosa), Euonymus alatus,
and Ligustrum sinense (Common Chinese Privet). An additional 17 were identified
as ones that may become widespread in the future. Families with the largest number
of species were Rosaceae, Caprifoliaceae, and Oleaceae. Most shrubs originated in
eastern Asia, were introduced in the 19th century, have animal-dispersed fruit, reproduce
vegetatively, are at least partially shade-tolerant, and tolerate a wide range of
soils. Many have extended-deciduous leaf habits and tolerate drought. Mechanical
and chemical control methods appear to be best for controlling them, but the use of
fire and biological control has been studied for only a few species. Invasive shrub
impact is difficult to assess from distribution alone, and further work is needed to
determine current and future impact of the species identified in this study.
Introduction
Invasive plant species are now recognized as a major problem in ecosystems
across the US, including those in Kentucky. Many of them crowd
out native species, including the ones that are desired for regeneration after
disturbance. Others may change forest ecosystem function in such a way that
successful tree regeneration no longer occurs. In the past, invasive species
were viewed as occupying niches created by human disturbance of natural
ecosystems (e.g., Mooney and Drake 1989, Rejmánek 1989). However, more
recent work has shown that relatively undisturbed ecosystems can be successfully
invaded (e.g., Gordon 1998, Horvitz et al. 1998). This vulnerability
to invasion is due to characteristics possessed by most successful invaders,
including 1) a high rate of reproduction and dispersal, 2) superior competitive
ability, 3) few herbivores or diseases, 4) an ability to occupy a “vacant” niche,
and 5) the capability of altering site characteristics (Gordon 1998).
For the purposes of this study, I define any shrub species that has been
called non-native, naturalized, alien, exotic, or introduced as an invasive
species. Some reserve the term “invasive” for introduced plants that are actively
expanding their range and abundance. However, many alien plants that
escape from cultivation undergo a substantial “lag” phase before their spread
*Department of Biological Sciences, Northern Kentucky University, Nunn Drive,
Highland Heights, KY 41099; boycer@nku.edu.
2 Northeastern Naturalist Vol. 17, Monograph No. 7
begins (Mack et al. 2000). Thus, non-native plants that may appear to be wellbehaved
at present are included in this study, as they may be in their lag phase.
In this review, I will focus on the effects of invasive shrub species in Kentucky,
particularly on forest tree regeneration. Results from Kentucky can be
extrapolated to a fairly large region of the US, as its flora, physical geography,
and climate are similar to much of the seven states that border it. Furthermore,
whereas Kentucky is only a moderate-sized state, its 120 counties allow a
fine degree for resolution for species distribution. While shrub data directly
applicable to Kentucky ecosystems would be most useful, such data for most
species included in this study are rare, and so I also include data from other
regions. Shrubs can have particular effects on trees not caused by other plant
forms. They are relatively tall and long-lived, so they compete against tree
seedlings longer than herbaceous vegetation. Miller (2005) has identified a
number of other effects. By forming monotypic stands, invasive shrubs reduce
biodiversity and hinder forest tree regeneration. This effect is clearly evident
where I live and work, in the Ohio River valley region, where the invasive
Lonicera maackii (Rupr.) Herder (Amur Honeysuckle) successfully keeps
the dominant oak-hickory forests from regenerating in many places, due to
its extended leaf-out period and the resulting dense shade (Luken and Thieret
1995), as well as its allelopathic effects on other plants (Cipollini et al. 2008,
Dorning and Cipollini 2006). Invasive shrubs can destroy habitat for other
organisms and disrupt important ecological processes, such as fire frequency
and intensity, nitrogen cycling, and soil pH equilibrium. Many invasive shrubs
take advantage of disturbance to initially become established. They tend to be
prolific seed and fruit producers, and their seed is spread by animals, wind,
and water. Seeds of many species can remain viable in the seed bank for many
years. Invasive shrubs often also reproduce vegetatively, and they can come
to dominate a stand via the production of root sprouts, stem sprouts, and other
forms of vegetative reproduction.
The objective of this review is to survey the extent of invasive shrubs in
Kentucky. I look at shrub distribution, nativity, physiological characteristics,
habitat preferences in the US, effects on forest trees (if known), and control
measures. Recommendations for recognition and control of current and potential
invasive shrubs follows, as well areas that require further research.
Methods
Data for this study were collected primarily from the USDA PLANTS
database (USDA NRCS 2008) and EDDMapS (2009), with additional references
cited in Supplemental Table 1 (available online at http://www.
eaglehill.us/NENAonline/suppl-files/n17-2-Boyce-s1, and, for BioOne
subscribers, at http://dx.doi.org/10.1656/N840.s1). Invasive species were
included only if they were listed in at least one Kentucky county by USDA
PLANTS, EDDMapS, or other sources. In addition, the growth habit listed in
the USDA PLANTS database had to include the term “shrub,” i.e., a perennial,
multi-stemmed woody plant that is usually less than 4 to 5 m in height (USDA
2010 R.L. Boyce 3
NRCS 2008). Exceptions were made for species included in the genera Rosa
and Rubus. In the USDA PLANTS database, they are not listed as shrubs,
but rather as subshrubs, i.e., low-growing shrubs less that 0.5 m tall, never
exceeding 1 m. However, other references included in this study state that
they all reach more than 1 m in height, and thus they are included. Maclura
pomifera (Raf.) C.K. Schneid (Osage-orange) was not included because it
grows as a tree in Kentucky. Species nomenclature follow USDA PLANTS
(USDA NRCS 2008). The 2004 estimated Kentucky county human population
data were obtained from the US Census Bureau (2005), and total number
of invasive shrub species in each county was regressed against the logarithm
of county population. I hypothesized that counties with herbaria, and those
adjacent, would have better invasive shrub collections than other counties due
to the presence of botanists adding to herbaria collections. Thus, a multiple
regression of species number in each county was performed on both the logarithm
of county population and a dummy variable that was set to either 1, if the
county included or was adjacent to an herbarium, or 0 otherwise. Because this
analysis indicated that invasive shrub species presence was undercounted in
counties away from herbaria, quantile regression was also performed, using
the statistical package R (R Development Core Team 2008), to better estimate
the most likely occurrence of invasive shrubs. In addition to tallying the total
number of counties where each invasive species occurred, I also collected and
tabulated physiological characteristics and habitat preferences, effects on forests,
control measures, and nativity.
In order to assess the potential of species that are currently not widespread
in Kentucky to become so, I performed a fuzzy set ordination (FSO) on the
data set (Boyce and Ellison 2001; Roberts 1986, 2008). A number of species
characteristics were used to construct a similarity matrix among species.
These characteristics were: origins from either eastern Asia or western Eurasia;
open-canopy forest habitat; riparian habitat; a heavy, conspicuous, or
persistent fruit crop; distribution of seed by birds; vegetative reproduction;
shade tolerance; leaf habit (deciduous, extended-deciduous, or evergreen);
drought tolerance; and nitrogen fixation. Most of these factors were binary
(0 or 1); shade and leaf habit were assigned three levels. Ružička’s index was
used to calculate similarities (Boyce and Ellison 2001), as it gave the best
fit. FSO was performed on the species similarity index, using the number of
counties (breadth of occurrence) as the factor. Ordination scores were then normalized
to the same range as the actual number of counties, i.e., the apparent
number of counties. The minimum apparent number of counties for widespread
species (actual number of counties ≥40) were then calculated; other
species that had apparent number of counties scores equal to or higher than this
minimum were classified as of concern for becoming widespread in the future.
Results
Number and distribution
Sixty-eight invasive shrub species occur in Kentucky (Appendix 1). The
occurrences in the 120 counties in Kentucky are listed in Supplemental
4 Northeastern Naturalist Vol. 17, Monograph No. 7
Figure 1. Number of invasive
shrubs species vs. logarithm
of estimated 2004 Kentucky
county populations. The
light dashed line shows the
results of a linear regression
(No. Spp. = -15.091 +
4.873 x log10[2004 county
population]; R2 =0.1472, P <
0.0001). The inclusion of a
dummy variable for the presence
of an herbarium in the
county or adjacent to it resulted
in the following multiple
linear regression equation:
No. Spp. = - 12.8727 +
4.1834 x log10(2004 county
population) + 2.0307 x (Herbarium
presence [1 or 0]);
R2 = 0.1886, Herbarium presence P = 0.0160. The heavy dashed line and the solid
line show the 90% and 95% quantile regression lines (No. Spp. = -18.148 + 7.040 x
log10[2004 county population] and No. Spp. = -15.913 + 6.978 x log10[2004 county
population], respectively) and are discussed in the text. All labeled counties (except
Jefferson) have active herbaria.
Table 1 (available online at https://www.eaglehill.us/NENAonline/supplfiles/n17-2-Boyce-s1, and, for BioOne subscribers, at http://dx.doi.
org/10.1656/N840.s1). An additional 6 species are listed by Jones (2008) as
occurring in Kentucky, but I was either unable to find county occurrences
and/or there is some doubt as to whether they are spreading from introduced
sites (Jones 2008); these include Ribes aureum Pursh var. villosum
DC. (Golden Currant), R. rubrum L. (Cultivated Currant), Rosa gallica L.
(French Rose), R. rugosa Thunb. (Rugosa Rose), Spiraea thunbergii Siebold
ex Blume (Thunberg’s Meadowsweet), and Tamarix parviflora DC. (Smallflower Tamarisk). The most widespread species (≥40 counties) are Rosa
multiflora (Multiflora Rose; 84), Elaeagnus umbellata (Autumn Olive; 52),
Morus alba (Mulberry; 48), Lonicera maackii (Amur Honeysuckle; 47), Albizia
julibrissin (Mimosa; 46), Euonymus alatus (46), and Ligustrum sinense
(Common Chinese Privet; 40).
The number of invasive shrub species showed a linear increase with the
logarithm of Kentucky county population (Fig. 1). Six counties with active
herbaria (Calloway, Campbell, Fayette, Madison, Rowan, and Warren) are
found well above the linear regression line. Jefferson County, which contains
Louisville and is Kentucky’s most populous county, is also above the
regression line. A multiple linear regression with a dummy variable for herbarium
presence in or adjacent to the county also showed significance (P =
0. 0160), indicating that invasive shrubs are more likely to be undercounted
in counties remote from herbaria, i.e., most of Kentucky. The 0.90 and 0.95
2010 R.L. Boyce 5
quantile regression lines include 90 and 95%, respectively, of the data points
under them (Fig. 1). Most of the points that lie close to the quantile regression
lines are for counties with or near herbaria or with recently published
checklists (data not shown). Thus, these lines are likely to be truer estimates
of shrub abundance than the linear regression line.
Taxonomy
Shrubs from the families Rosaceae have been the most successful in invading
Kentucky (18 species), followed by Caprifoliaceae (14) and Oleaceae
(8) (Fig. 2). The large number of species in the genera Rosa, Lonicera, and
Ligustrum are responsible for these trends (Appendix 1). Elaeagnaceae
and Rhamnaceae have 4 and 3 species, respectively. Aceraceae, Celastraceae,
Hydrangeaceae, Salicaceae, and Verbenaceae each have 2. The
families Aquifoliaceae, Araliaceae, Berberidaceae, Cupressaceae, Fabaceae,
Grossulariaceae, Hippocastanaceae, Malvaceae, Moraceae, Taxaceae, and
Ulmaceae are each represented by 1 species.
Timeframe of introduction
A few shrubs (13) were introduced into the US before 1800 (Appendix 1).
However, most (45) were introduced in the 19th and 20th centuries—with introductions
likely still occurring now in the 21st century. Despite recognition
Figure 2. Classification of Kentucky invasive shrub species by family.
6 Northeastern Naturalist Vol. 17, Monograph No. 7
of the problem of invasive species, a large number—88%—of these shrubs
are still distributed by vendors in the US (Appendix 1). Virtually all of these
species can be purchased on the web from international vendors.
Native ranges of exotic shrubs
Most invasive shrubs in the US are from two regions of the world—
eastern Asia and western Eurasia (including Europe, north Africa, and
western Asia; Fig. 3). Kentucky has a climate similar to eastern Asia, so it
is unsurprising that Asian shrubs do well in this part of the country. Europe
is well-represented because US gardeners often follow European gardening
traditions and use European plants, and the Kentucky climate is similar
enough to that of Europe for many European shrubs to survive.
Habitats in the US
Most invasive shrubs in Kentucky live in a variety of different habits
in the US (and therefore presumably in Kentucky); the specific habitats
are described in Supplemental Table 2 (available online at http://www.
eaglehill.us/NENAonline/suppl-files/n17-2-Boyce-s1, and, for BioOne
subscribers, at http://dx.doi.org/10.1656/N840.s1). By grouping these
habitats into broad categories, however, we can see what kinds of habitats
invasive shrubs generally select. More than 95% invade disturbed and/or
open habitats (Fig. 4). This finding is not surprising, given that invasive
plants are often found in resource-rich disturbed habitats (e.g., Drake et al.
1989, Hobbs and Huenneke 1992). Almost half also invade riparian zones,
probably because these areas often are also disturbed and are rich in other
resources. Almost two-thirds will invade open-canopy forests, while less
Figure 3. Regions of
the world where Kentucky
invasive shrubs
are native.
2010 R.L. Boyce 7
than 1/4 can invade closed-canopy forest, which appears to be due to differences
in shade tolerance (see below).
Physiological characteristics
Seed reproduction and vectors. More than three-quarters of invasive
shrubs have a heavy, conspicuous, or persistent fruit crop (Fig. 5),
which is attractive to a variety of animals (Fig. 6; see also Supplemental
Table 3, available online at https://www.eaglehill.us/NENAonline/
suppl-files/n17-2-Boyce-s1, and, for BioOne subscribers, at http://
dx.doi.org/10.1656/N840.s1). While most of the other shrubs for which
data are available do not produce attractive fruits, they do produce heavy
seed crops. These findings are not surprising, because many of these shrubs
have escaped from gardens, where they are often grown for their flowers,
fruit, or both, and gardeners usually prefer plants that bloom and/or yield
prolifically. It is common for shrubs to produce large numbers of small
fruits, so birds are responsible for spreading more than 30 of these species
(Fig. 6). A number of other animals also spread these plants. Wind, human
activities, water, and seed are also responsible for spreading some species.
Vegetative reproduction. Almost three-quarters (47 of 68) of these species
can reproduce vegetatively or asexually (Supplemental Table 3, available online
at https://www.eaglehill.us/NENAonline/suppl-files/n17-2-Boyce-s1,
and, for BioOne subscribers, at http://dx.doi.org/10.1656/N840.s1). This
trait allows some species to dominate an area more quickly than would be pos-
Figure 4. Habitat type in which Kentucky invasive shrubs can grow. Many species
can grow in more than one habitat type, and thus percentages total to more than
100%.
8 Northeastern Naturalist Vol. 17, Monograph No. 7
Figure 5. Sexual reproduction
modes used
by Kentucky invasive
shrubs. “Fruit crop”
means an attractive
fruit that is consumed
by animals, whereas
“seed crop” means that
fruits are not usually
consumed, and therefore
seeds are spread
by other vectors. ND =
no data. Species with
multiple fruit classifi-
cations were placed in
the “heavy fruit crop”
category.
Figure 6. Dispersal agents used by Kentucky invasive shrubs. Many species use more
than one agent. ND = no data.
2010 R.L. Boyce 9
sible if they relied only on sexual reproduction. It also means that special care
needs to be taken during control, as plant parts left on a site may regenerate.
Shade tolerance. A total of 46 invasive shrub species are partially shade
tolerant (Fig. 7, Appendix 2), allowing them to invade partially open, undisturbed
forests, as well as more open areas. Eight species are intolerant and
are restricted to open and/or disturbed areas. Only 13 species are considered
to be shade tolerant; these species often have the ability to invade closedcanopy
forest.
Leaf habit. More than half of invasive shrubs are classified as deciduous
(Fig. 8, Appendix 2). Few are evergreen, but many (≈30%) have an extended
leaf period, i.e., they leaf out early and/or retain leaves longer than native
woody plants. In the literature, these plants are referred to by a variety of
terms, including semi-deciduous, tardy-deciduous, extended-deciduous, and
semi-evergreen; here, I classify them as “deciduous-extended”.
Soil and water requirements. Since invasive shrubs grow in a wide variety
of habitats, it is not surprising that soil and water requirements are quite
variable (Appendix 2). More than 95% grow on what is defined as a “wide
range” of soil types, which may aid in the initial establishment of invasive
species. While water requirements are more variable, close to one-third grow
under a wide range of availabilities, and almost 15% are drought tolerant or
resistant (Fig. 9). This plasticity in water requirements no doubt also aids in
the initial establishment of these species.
Effects
Almost all shrubs can directly suppress other plants via competition,
so here I focus on more indirect effects of invasive shrubs. The indirect
Figure 7. Shade tolerance
of Kentucky invasive
shrubs. ND =
no data.
10 Northeastern Naturalist Vol. 17, Monograph No. 7
effects of more than one-third of these shrubs have not been determined
(Fig. 10; Supplemental Table 4, available online at http://www.eaglehill.
us/NENAonline/suppl-files/n17-2-Boyce-s1, and, for BioOne subscribers,
at http://dx.doi.org/10.1656/N840.s1); clearly, much more study is required.
Some of the common indirect effects include decreased community richness,
alteration of nutrient cycling, allelopathy, competition for pollinators, and
restriction of wildlife use (Fig. 10; Supplemental Table 4, available online
at https://www.eaglehill.us/NENAonline/suppl-files/n17-2-Boyce-s1, and,
for BioOne subscribers, at http://dx.doi.org/10.1656/N840.s1). Some species
can change (usually enhance) soil N, an ability common to all the N-fixing
species, i.e., all members of Elaeagnaceae and Fabeaceae (Appendix 2). Other
effects include being a host for diseases that affect other plants, changes in fire
regimes, and interbreeding with native species (Fig. 10).
Control
Physical control. The most effective form of physical control involves
uprooting (Fig. 11, Appendix 3). This approach is used most often on young
shrubs, as they are easier to uproot. It is also easier to extract their whole
root systems, which is important because most of the shrubs for which data
are available will resprout after cutting (Appendix 3). Cutting alone is effective
on only about one-quarter of all species (Fig.11) and often needs to be
repeated. The effectiveness of physical control for a substantial fraction of
invasive shrubs has not been determined.
Fire control. More than two-thirds of the species have not been evaluated
(Fig. 12, Appendix 3). Of the ones that have, only a few show complete
Figure 8. Leaf habit
of Kentucky invasive
shrubs. Deciduous-
extended
includes a variety
of terms including
semi-evergreen or
semi-deciduous.
Deciduous or evergreen
includes
plants that can exhibit
either strategy,
depending on location.
2010 R.L. Boyce 11
Figure 9. Water requirements
of Kentucky
invasive shrubs.
ND = no data.
Figure 10. Indirect effects of Kentucky invasive shrubs. ND = no data.
12 Northeastern Naturalist Vol. 17, Monograph No. 7
control by fire alone, and that is only if it is repeated. More studies have
reported that fire control is partially effective, and it often works best when
combined with other treatments (Appendix 3). Some species, of course, are
actually stimulated by fire, either through resprouting, heavy seed germination,
or a combination.
Chemical control. Control with herbicides is currently the most effective
way to control invasive shrubs, 40 of which can be controlled by the
application of at least one herbicide (Appendix 3). Effectiveness has still not
been determined on 28 out of the 68 species, however. Herbicides are often
combined with cutting, where herbicide is painted or sprayed onto freshly
cut stumps. Specific herbicides and their methods of application will not be
discussed here, but can be found in the cited references. Some of the most effective
herbicides, e.g., glyphosate, are broad-spectrum and will kill desired
species as well, and so they must be used carefully. Land managers may also
hesitate to use a chemical agent for an infestation over a wide area, due to possible
detrimental environmental effects and costs of herbicide applications.
Biological control. More than half of the shrub species have not been
evaluated (Fig. 13, Appendix 3). Grazing animals, such as goats, sheep,
cattle, and chickens, have shown some promise for controlling a few species.
Six species show potential for control by biological agents, while no biological
agent has been found so far for 13 species.
Species likely to become widespread
All of the eight most widespread species have high ordination scores,
i.e., high apparent number of counties (Appendix 1, Fig. 14). The FSO is
statistically significant (P = 0.0135), showing that shrub occurence is correlated
to the suite of physiological characteristics chosen for the similarity
Figure 11. Physical
or mechanical control
methods that
have been found
to be effective in
controlling shrubs
that are invasive in
Kentucky.
2010 R.L. Boyce 13
matrix. A total of 17 other species also have high ordination scores, higher
than the minimum of the seven species currently most widespread. These
species are Aesculus parviflora, Berberis thunbergii, Crataegus monogyna,
Elaeagnus angustifolia, E. pungens, Kerria japonica, Ligustrum ovalifolium,
Figure 12. Fire control
methods for shrubs
that are invasive in
Kentucky.
Figure 13. Types
of biological
control agents
found for Kentucky
invasive
shrubs. NK =
report that none
are known. ND
= no report of
either an effective
agent or a
lack of one.
14 Northeastern Naturalist Vol. 17, Monograph No. 7
L. vulgare, Lonicera tatarica, Lonicera x bella, Rhamnus cathartica, Rhodotypos
scandens, Rubus armeniacus, R. phoenicolasius, Salix purpurea,
Ulmus pumila, and V. opulus var. opulus. Species with high ordination scores
are similar to widespread species, while not similar to less commonly occurring
species (Roberts 1986). Thus, these 17 species have characteristics of
species already widespread and could in turn become widespread (found in
≥40 counties) over time. As these species increase their range, the strength
of their ordination scores is expected to increase.
Discussion
The number of invasive shrubs in Kentucky is substantial; there are 68
with good documentation, while there are probably others that have not yet
Figure 14. Fuzzy set ordination (FSO) of invasive shrub species in Kentucky. The
similarity matrix used in the ordination was drawn from invasive shrub characteristics,
as described in the text, and the factor used in the ordination was the number of
counties in which the species were found. The ordination is statistically significant
(Spearman r = 0.298, P = 0.014). Points with abbreviations (first two letters of generic
and specific epithets) and in boldface indicate species already widely distributed.
The 17 points with abbreviations in plain text indicate species with high normalized
ordination scores (apparent number of counties) that are greater than the minimum
ordination score for already-widespread species. Note that three species are clustered
tightly together.
2010 R.L. Boyce 15
been recognized. The USDA PLANTS Database (USDA NRCS 2008) lists
some of the species included here as having life forms of both a shrub and
a tree. It is often difficult to decide exactly how to classify larger woody
plants, as some can grow as a tree (i.e., with a single stem) under some circumstances,
while they can grow as a shrub (i.e., with many stems) under
others. For example, Morus alba can occur as a tree as well as a shrub in
Kentucky (R.L. Boyce, pers. observ.). Some of the species listed here were
not described in the recent catalog for Kentucky woody plants by Clark and
Weckman (2008). However, even with the more liberal criteria employed
in the current study, it is clear that that invasive shrubs are undercounted in
most regions of the state. Madison County is home to Eastern Kentucky University
and Berea College, both with very active taxonomists (Ross Clark,
Ron Jones, Ralph Thompson, and Timothy Weckman), and it lies well above
even the quantile regression lines (Fig. 1). Jefferson County, which includes
Louisville, Kentucky's second largest city, also lies near the quantile regression
lines. The University of Louisville no longer has an active herbarium,
but its collection was incorporated into the Western Kentucky University
Herbarium (SERNEC 2010). In addition, many academic institutions as well
as natural resource agencies are located in Louisville, and professionals from
these organizations may have reported sightings to the USDA PLANTS and
EDDMapS databases. Thus, even these quantile regressions appear to be underestimates
of the true number of invasive shrubs that occur in Kentucky.
The most well-represented families of invasive woody plants in the
Czech Republic were Rosaceae, Caprifoliaceae, and Oleaceae, as they are
in the current study (Křivánek and Pyšek 2008). These three families also
account for 12 of the 22 invasive shrub species listed on the IPANE database
for New England (Mehrhoff et al. 2004). Frappier and Eckert (2003) found
that the factors which were the best predictors of invasiveness for woody
plants in New Hampshire were resprouting ability, lower shade tolerance,
tolerance of low soil fertility, and being an angiosperm. Herron et al. (2007)
also found that invasive shrubs in New England were not evergreen and
did not have wind-dispersed seeds; however, there was no association with
shade tolerance nor possession of bird-dispersed fruits. The results from
the current study also show strong associations with deciduousness/nonevergreenness,
resprouting, and tolerance of low soil fertility. While some
of the other traits, such as bird-dispersed fruits and lower amounts of shade
tolerance, may also be important predictors of invasiveness in Kentucky,
Herron et al. (2007) cautioned that these traits may not sufficiently distinguish
invasive shrubs from non-invasive ones.
Some of the included species are not currently having much impact. However,
lag phases for introduced shrubs can be quite long. Křivánek and Pyšek
(2008) showed that the average lag phase for invasive shrubs in the Czech Republic,
i.e., the time between introduction and escape, was 110 years, ranging
from 20 to 257 years. As a large proportion of the species listed here have been
present in Kentucky, with a similar temperate climate, for that time or less,
16 Northeastern Naturalist Vol. 17, Monograph No. 7
even species with very restricted distributions should be closely monitored, as
they may still be in the lag phase. The FSO suggests that 17 species may currently
be in lag phases; these species should be monitored closely and perhaps
be treated most aggressively at present. Some of these species, such as Berberis
thunbergii, Elaeagnus spp., Lonicera spp., and Rhodotypos scandens, are
already recognized as major problems in other states. Others, such as Aesculus
parviflora and Kerria japonica, are not, but they do have the same set of characteristics
as species already widespread in Kentucky.
It is clear that the most heavily populated areas of the state are serving
as sources of invasive shrubs, probably most of which have escaped from
cultivation. Thus, we need both a better tally of those regions of the state that
have not been well-surveyed, as well as closer monitoring of more populated
regions that are serving as sources for new introductions.
One of the first steps needed to deal with invasive shrubs is to recognize
the scope of the problem. Some states, notably Florida (Invasive Species
Working Group; http://iswgfla.org/) and California (Department of Fish and
Game; http://www.dfg.ca.gov/invasives/), have state agencies that monitor
the presence and spread of invasive species. Other states, such as Tennessee
(Tennessee Exotic Pest Plant Council; http://www.tneppc.org/), have wellestablished
nongovernmental organizations that play a similar role. The
University of Montana INVADERS database (http://invader.dbs.umt.edu/)
plays a similar role in five northwestern states. Kentucky is a member of the
Southeast Exotic Pest Plant Council (http://www.se-eppc.org/) and has an
active state chapter (http://www.se-eppc.org/ky/). This organization needs
to be supported in its efforts to monitor and control the spread of invasive
plants in Kentucky.
State (and federal) governments need to consider greater regulation of
plants that are both known and suspected to be invasive. Most of the shrubs
described in this study are known to be invasive yet can be purchased on
the web and/or local nurseries. While Kentucky and many other states do
have noxious-plant laws, few invasive shrubs are included. For example,
only Rosa multiflora is on Kentucky’s list (Kentucky Revised Statutes
1990). Thus, vendors and purchasers are unlikely to know that they are
dealing with invasive plants. Clearly, both vendors and consumers need to
be educated about invasive plants. Stronger regulations, both at the state
and federal levels, would also help control the spread of invasive shrubs
in particular and invasive plants in general. We may also want to consider
regulating plants that currently are not widespread, but have traits common
to invasives that may make them problematic in the future. From this
survey, it is clear that most invasive shrubs in Kentucky share a number of
properties, including resprouting ability, partial shade tolerance, a heavy
fruit crop that is attractive to birds, and extended deciduous or deciduous
habit. Certain families, such as Rosaceae, Caprifoliaceae, and Oleaceae,
also contain a large number of invasive shrubs and thus should be regarded
with suspicion. Not all of these properties may be good predictors of invasiveness,
but shrubs that fall in several of these categories appear to be at
2010 R.L. Boyce 17
high risk of becoming invasive, and regulation may be merited before these
shrubs become a problem.
More research of all kinds is also needed for the invasive shrubs that
we do recognize at present. The physiological requirements of many species
are known either poorly or not at all. The potential habitats of some
shrubs also need more study. Clearly, much more work needs to be put into
discovering better control methods. Both fire and biological control methods
merit much more work than they have received to date. Also, more
work needs to be done on the direct and indirect effects of invasive shrubs.
We know that in many cases, invasive shrubs displace or prevent the establishment
of native tree species. It is clear, however, that shrubs do this
in a variety of ways (e.g., Gordon 1998). It is also clear that they can have
numerous indirect effects on forest ecosystems (e.g., Horvitz et al. 1998).
Knowledge of these effects is critical if we are to successfully control invasive
species and restore native forests.
Other steps need to be taken as well (Webster et al. 2006). This study
shows that certain shrubs, such as Rosa multiflora and Elaeagnus umbellata,
are quite widespread. However, a wide distribution does not always
translate into a large impact, especially if shrub density remains low. Local
shrub density is probably a better indication of impact to local ecosystems,
but these data are generally lacking. An enhanced understanding of their
local impact would allow us to rank shrubs by the threat they represent and
would allow control methods to be prioritized. Because timber harvests
and other human activities that disturb forests aid in the spread of many of
these invasive shrubs, invasive treatment and monitoring efforts need to be
included in these activities. This approach will also mean more cooperation
between public and private sectors. Finally, the best control methods need to
be communicated and used.
Acknowledgments
I would like to thank Maggie Whitson, both for permission to use the John W.
Theriet Herbarium at Northern Kentucky University and for her extraordinary job of
reviewing this manuscript.
Literature Cited
ABC. 2008. Gardening Australia – Fact Sheet: Pete’s Garden. Available online at http://
www.abc.net.au/gardening/stories/s1298518.htm. Accessed 14 January 2010.
Adams, D.W. 2004. Restoring American Gardens: An Encyclopedia of Heirloom
Ornamental Plants, 1640–1940. Timber Press, Portland, OR. 420 pp.
Albrecht, L.A. 2001. Jetbead: A new invasive threat. Northeastern Weed Science Society
Newsletter. April 2001:7. Available online at http://www.newss.org/docs/
newsletter/2001_nl_apr.pdf. Accessed 14 January 2010.
Alverson, E., and J. Sigg. 2005. Crataegus monogyna. California Invasive Plant
Council (Cal-IPC). Available online at http://www.cal-ipc.org/ip/management/
ipcw/pages/detailreport.cfm@usernumber=37&surveynumber=182.php. Accessed
14 January 2010.
18 Northeastern Naturalist Vol. 17, Monograph No. 7
Amrine, J.W., Jr. 2002. Rosa multiflora. Pp. 265–292, In R. Van Driesche, S. Lyon, B.
Blossey, M. Hoddle, and R. Reardon (Eds.). Biological Control of Invasive Plants
in the Eastern United States. USDA Forest Service Publication FHTET-2002-04,
Washington, DC. 430 pp. Available online at http://www.invasiveplants.net/biol
ogicalcontrol/22MultifloraRose.html. Accessed 14 January 2010.
Anderson, E.D. 2007. IPA targets invasive gray willow for removal. The IPA Newsletter
7(1):1. Available online at http://www.indianponds.org/2007%20Winter%
20Newsletter.pdf. Accessed 14 January 2010.
BackyardGardener. 2010. Garden Plant Index – Flora – Botanical. Available online
at http://www.backyardgardener.com/plantname/index.html. Accessed 14 January
2010.
Bargeron, C.T., D.J. Moorhead, G.K. Douce, R.C. Reardon, and A.E. Miller (Tech.
Coords.). 2003. Invasive Plants of the Eastern United States: Identification
and Control. The University of Georgia, USDA APHIS PPQ and USDA Forest
Service Forest Health Technology Enterprise Team. FHTET-2003-08. Available
online at http://www.invasive.org/eastern/. Accessed 14 January 2010.
Batcher, M.S. 2000. Element Stewardship Abstract for Ligustrum spp. 10 pp. Available
online at http://www.imapinvasives.org/GIST/ESA/esapages/documnts/
ligu_sp.pdf. Accessed 14 January 2010.
Batcher, M.S., and S.A. Stiles. 2000. Element Stewardship Abstract for Lonicera
maackii, Lonicera morrowii, Lonicera tatarica, Lonicera x bella. 11 pp. Available
online at http://www.imapinvasives.org/GIST/ESA/esapages/documnts/
loni_sp.pdf. Accessed January 14, 2010.
Bergmann, C., and J.M. Swearingen. 2009. PCA Alien Plant Working Group – Multiflora Rose (Rosa multiflora). Available online at http://www.nps.gov/plants/alien/
fact/romu1.htm. Accessed 14 January 2010.
Biosecurity New Zealand. 2008. Grey Willow. MAF Biosecurity New Zealand.
Available online at http://www.biosecurity.govt.nz/pests/grey-willow. Accessed
14 January 2010.
Boiteau, K., S. Leicht, and L. Mehrhoff. 2009. Northeastern National Parks Invasive
Evaluation Project. Available online at http://invasives.eeb.uconn.edu/ipane/
nationalparks/NPS.htm. Accessed 14 January 2010.
Boyce, R.L., and P.C. Ellison. 2001. Choosing the best similarity index when
performing fuzzy set ordination on binary data. Journal of Vegetation Science
12:711–720.
Brand, M.H. 2001. UConn Plant Database Main Page.htm. Available online at http://
www.hort.uconn.edu/plants/. Accessed 14 January 2010.
Browne, D.J. 1846. The Trees of America: Native and Foreign, Pictorially and
Botanically Delineated, and Scientifically and Popularly Described. Harper &
Brothers, New York, NY. 520 pp.
Brusati, E. 2005. Plant assessment form. Elaeagnus angustifolia. 8 pp. Available
online at http://www.cal-ipc.org/ip/inventory/PAF/Elaeagnus%20angustifolia.
pdf. Accessed 14 January 2010.
Bushes and Shrubs. 2003. Ligustrum amurense. Available online at http://www.
bushesandshrubs.com/scientific_names/ligustrum_amurense.shtml. Accessed 14
January 2010.
Canadian Botanical Conservation Network (CBCN). 2010. Invasive Shrubs and
Vines. Available online at http://www.rbg.ca/cbcn/en/projects/invasives/i_
shrub2.html. Accessed 14 January 2010.
2010 R.L. Boyce 19
Center for Earth and Environmental Science. 2005. Lilly Arbor Project. Data. Available
online at http://www.cees.iupui.edu/Research/Restoration/ARBOR/Data/
Lilly_Pad/Invasive_Exotic/White_Mulberry/White_Mulberry.doc. Accessed 14
January 2010.
Cheltenham Township. 2009. Invasive Species Management Program. Available
online at http://www.cheltenhamtownship.org/stormwater/INVASIVESPECIESPROGRAM.
htm. Accessed 14 January 2010.
Christman, S. 2008. FloridataBase Plant Browser. Available online at http://www.
floridata.com/FloridataBase/browseFloridata.cfm. Accessed 14 January 2010.
Cipollini, D., R. Stevenson, S. Enright, A. Eyles, and P. Bonello. 2008. Phenolic
metabolites in leaves of the invasive shrub, Lonicera maackii, and their potential
phytotoxic and anti-herbivore effects. Journal of Chemical Ecology
34:144–152.
Clark, R.C., and T.J. Weckman. 2008. Annotated catalog and atlas of Kentucky
woody plants. Castanea Occasional Papers in Eastern Botany No. 3:1–114.
Cochran, K.D. 2000. A history of yews in the United States, In J.A. Chatfield, G.Y.
Gao, E.A. Draper, and J.F. Boggs (Eds.). Ornamental Plants Annual Reports and
Research Reviews 2000. Special Circular 177-01. Available online at http://ohioline.
osu.edu/sc177/index.html. Accessed 14 January 2010.
Converse, C.K. 1984. Element Stewardship Abstract for Rhamnus cathartica,
Rhamnus frangula (syn. Frangula alnus). 14 pp. Available online at http://www.
imapinvasives.org/GIST/ESA/esapages/documnts/franaln.pdf. Accessed 14
January 2010.
Dana, M.N., and B.R. Lerner. 2006. Landscape plants for sandy soils. Purdue University
Cooperative Extension Service. Landscape Horticulture HO-225-W. 4
pp. Available online at http://www.hort.purdue.edu/ext/HO_225.pdf. Accessed
14 January 2010.
Deiter, L. 2005. Elaeagnus angustifolia. California Invasive Plant Council (Cal-
IPC). Available online at http://www.cal-ipc.org/ip/management/ipcw/pages/
detailreport.cfm@usernumber=46&surveynumber=182.php. Accessed 14 January
2010.
Dorning, M., and D. Cipollini. 2006. Leaf and root extracts of the invasive shrub,
Lonicera maackii, inhibit seed germination of three herbs with no autotoxic effects.
Plant Ecology 184:287–296.
Drake, J.A., H.A. Mooney, F. di Castri, R.H. Groves, F.J. Krueger, M. Rejmánek
and M. Williamson (Eds.). 1989. Biological Invasions: A Global Perspective.
Scientific Committee on Problems of the Environment (SCOPE) of the International
Council of Scientific Unions (ICSU). John Wiley and Sons, New York,
NY. 550 pp.
Early Detection and Distribution Mapping System (EDDMapS). 2009. EDDMapS
Southeast Exotic Pest Plant Council – Invasive Species Mapping Made Easy. Available
online at http://www.eddmaps.org/southeast/. Accessed 14 January 2010.
Eckardt, N. 1987. Element Stewardship Abstract for Rosa multiflora. 8 pp. Available
online at http://www.imapinvasives.org/GIST/ESA/esapages/documnts/rosamul.
pdf. Accessed 14 January 2010.
Ensbey, R. 2004. Noxious and Environmental Weed Control Handbook 2004–2005:
A Guide to Weed Control in Non-Crop, Aquatic and Bushland situations. NSW
(New South Wales) Agriculture, Orange, Australia. 76 pp. Available online at
http://www.lhccrems.nsw.gov.au/weeds_cd/extras/nox-weeds-handbook2004.
pdf. Accessed 14 January 2010.
20 Northeastern Naturalist Vol. 17, Monograph No. 7
Evans, E. 2003. Shrubs. North Carolina State University. Available online at http://
www.ces.ncsu.edu/depts/hort/consumer/factsheets/shrubs/index.html. Accessed
14 January 2010.
Faucon, P. 2005. Amur Privet (Ligustrum amurense). Available online at http://www.
desert-tropicals.com/Plants/Oleaceae/Ligustrum_amurense.html. Accessed 14
January 2010.
Forest Health Protection (FHP) Invasive Plants. 2008. Invasive species, NA—Forest
health protection. Available online at http://www.na.fs.fed.us/fhp/invasive_
plants/weeds/. Accessed 8 December 2008.
Forest Health Protection (FHP) Invasive Plants. 2010. Invasive Species, NA—Forest
Health Protection. Available online at http://www.na.fs.fed.us/fhp/invasive_
plants/weeds/. Accessed 14 January 2010.
Frappier, B., R.T. Eckert, and T.D. Lee. 2004. Experimental removal of the nonindigenous
shrub Rhamnus frangula (Glossy Buckthorn): Effects on native herbs
and woody seedlings. Northeastern Naturalist 11:333–342.
Frappier, B., and R.T. Eckert. 2003. Utilizing the USDA PLANTS database to predict
exotic woody plant invasiveness in New Hampshire. Forest Ecology and Management
185:207–215.
Germplasm Resources Information Network (GRIN). 2009. Search GRIN for Taxonomy
of Plants. USDA Agricultural Research Service. Available online at http://
www.ars-grin.gov/cgi-bin/npgs/html/tax_search.pl. Accessed 14 January 2010.
Gooseberry Limited. 2010. Gooseberry Limited. Available online at http://uvacrispa.
com/. Accessed 14 January 2010.
Gorchov, D.L., and D.E. Trissel. 2003. Competitive effects of the invasive shrub,
Lonicera maackii (Rupr.) Herder (Caprifoliaceae), on the growth and survival of
native tree seedlings. Plant Ecology 166:13–24.
Gordon, D.R. 1998. Effects of invasive, non-indigenous plant species on ecosystem
processes: Lessons from Florida. Ecological Applications 8:975–989.
Gould, A.M.A., and D.L. Gorchov. 2000. Effects of the exotic invasive shrub Lonicera
maackii on the survival and fecundity of three species of native annuals.
American Midland Naturalist 144:36–50.
Hedgerowmobile. 2010. Species list; plant. Available online at http://hedgerowmobile.
com/specieslistplants.html. Accessed 14 January 2010.
Heidorn, R. 1990. Vegetation management guideline: Exotic buckthorns. Illinois Nature
Preserve Commission. Available online at http://dnr.state.il.us/INPC/VMG/
VMG%20Buckthorns%20original%201990.pdf. Accessed 14 January 2010.
Herron, P.M., C.T. Martine, A.M. Latimer, and S.A. Leicht-Young. 2007. Invasive
plants and their ecological strategies: Prediction and explanation of woody plant
invasion in New England. Diversity and Distributions 13:633–644.
Hobbs, R.J., and L.F. Huenneke. 1992. Disturbance, diversity, and invasion: Implications
for conservation. Conservation Biology 6:324–337.
Hoch, W.A. 2001. Landscape plants of the Upper Midwest: Black jetbead (Rhodotypos
scandens). Available online at http://www.midwestlandscapeplants.org/
plantdetails.cfm?speciesid=810. Accessed 14 January 2010.
Holst, P., and H. Simmonds. 2001. Chapter J1. Weed control using goats, In A.J.
Simmonds (Ed.). Australian Goat Notes. Australian Cashmere Growers Association,
Kellyville, NSW, Australia. 267 pp. Available online at http://acga.org.au/
goatnotes/J001.php. Accessed 14 January 2010.
Horticopia. 2004. Ligustrum obtusifolium. Available online at http://www.horticopia.
com/hortpix/html/pc3342.htm. Accessed December 8, 2008.
2010 R.L. Boyce 21
Horvitz, C.C., J.B. Pascarella, S. McMann, A. Freedman, and R.H. Hofstetter. 1998.
Functional roles of invasive non-indigenous plants in hurricane-affected subtropical
hardwood forests. Ecological Applications 8:947–974.
Hoshovsky, M. 1989. Element Stewardship Abstract for Rubus discolor, (Rubus
procerus). 12 pp. Available online at http://www.imapinvasives.org/GIST/ESA/
esapages/documnts/rubuarm.PDF. Accessed 14 January 2010.
Hoshovsky, M. 2005. Rubus discolor. California Invasive Plants Council (Cal-IPC).
Available online at http://www.cal-ipc.org/ip/management/ipcw/pages/detailreport.
cfm@usernumber=71&surveynumber=182.php. Accessed 14 January 2010.
Hutchinson, T.F., and J.L. Vankat. 1997. Invasibility and effects of Amur Honeysuckle
in southwestern Ohio forests. Conservation Biology 11:1117–1124.
Invasive Plant Atlas of the MidSouth (IPAMS). 2010. IPAMS—Species information:
Elaeagnus pungens—Thorny Olive. GeoResources Institute, Mississippi
State University. Available online at http://www.gri.msstate.edu/ipams/Species.
php?SName=&CName=Thorny+olive. Accessed 14 January 2010.
Invasive Species. 2009. Siberian Elm: Ulmus pumila (Urticales: Ulmaceae). Available
online at http://www.invasive.org/browse/subject.cfm?sub=3479. Accessed
14 January 2010.
Invasive Species Specialist Group (ISSG). 2010. ISSG's global invasive species
database. Available online at http://www.invasivespecies.net/database/welcome/.
Accessed 14 January 2010.
Johnson, F.L., and B.W. Hoagland. 1999. Catalog of the woody plants of Oklahoma:
Descriptions and range maps. Oklahoma Biological Survey. Available online at
http://www.biosurvey.ou.edu/shrub/shrubndx.htm. Accessed 14 January 2010.
Jones, R.L. 2008. Checklist of Kentucky Vascular Plants. Department of Biological
Sciences: Ronald L. Jones. Available online at http://people.eku.edu/jonesron/
checklist.php. Accessed 14 January 2010.
Kentucky Revised Statutes. 1990. Department to eradicate noxious weeds on right-ofways—
advertisement of program. Commonwealth of Kentucky. Available online
at http://www.lrc.state.ky.us/KRS/176-00/051.PDF. Accessed 14 January 2010.
King County Noxious Weed Control Program. 2005. Best Management Practices.
Evergreen blackberry (Rubus laciniatus) and Himalayan blackberry (Rubus discolor
syn. Rubus armeniacus). 7 pp. Available online at http://your.kingcounty.
gov/dnrp/library/water-and-land/weeds/BMPs/blackberry-control.pdf. Accessed
14 January 2010.
Kline, N. 2010. Invasive Exotic Plant Tutorial – Guelder rose. Pennsylvania DCNR
Invasive Exotic Plant Tutorial for Natural Lands Managers. Available online
at http://www.dcnr.state.pa.us/forestry/invasivetutorial/guelder_rose.htm. Accessed
14 January 2010.
Kling, G.J. 2010. UIPLANTS database. University of Illinois at Urbana-Champaign.
Available online at http://woodyplants.nres.uiuc.edu/. Accessed 14 January 2010.
Křivánek, M., and P. Pyšek. 2008. Forestry and horticulture as pathways of plant
invasions: A database of alien woody plants in the Czech Republic. Pp. 21–38, In
B. Tokarska-Guzik, J.H. Brock, G. Brundu, L. Child, C.C. Daehler, and P. Pyšek
(Eds.). Plant Invasions: Human Perception, Ecological Impacts and Management.
Backhuys Publishers, Leiden, The Netherlands. 400 pp.
Langeland, K.A., and K.C. Burks (Eds.). 1998. Identification and Biology of Non-
Native Plants in Florida's Natural Areas. University of Florida, Gainesville.
165 pp. Available online at http://www.fleppc.org/ID_book.htm. Accessed 14
January 2010.
22 Northeastern Naturalist Vol. 17, Monograph No. 7
Langeland, K.A., and R.K. Stocker. 2001. Control of Non-Native Plants in Natural
Areas of Florida. Department of Agronomy, Florida Cooperative Extension Service,
Institute of Food and Agricultural Sciences, University of Florida. 34 pp.
Available online at http://edis.ifas.ufl.edu/pdffiles/wg/wg20900.pdf. Accessed 14
January 2010.
Luken, J.O., and J.W. Thieret. 1995. Amur Honeysuckle (Lonicera maackii; Caprifoliaceae):
Its ascent, decline, and fall. Sida 16:479–503.
Mack, R.N., D. Simberloff, W.M. Lonsdale, H. Evans, M. Clout, and F.A. Bazzaz.
2000. Biotic invasions: Causes, epidemiology, global consequences, and control.
Ecological Applications 10:689–710.
Martin, T. 2005. TNC Invasive Species Initiative page. Weed Alert! Euonymus
alatus. Available online at http://www.invasive.org/gist/alert/alrteuon.html. Accessed
14 January 2010.
Mehrhoff, L.J., J.A. Silander, Jr., S.A. Leicht, E.S. Mosher, and N.M. Tabak. 2004.
IPANE: Invasive Plant Atlas of New England. Department of Ecology and Evolutionary
Biology, University of Connecticut, Storrs, CT, USA. Available online
at http://nbii-nin.ciesin.columbia.edu/ipane/. Accessed 14 January 2010.
Michigan State University (MSU) Extension. 1999. Chaenomeles speciosa--Flowering
Quince. Ornamental Plants plus Version 3.0 – 00000335. Available online at http://
web1.msue.msu.edu/imp/modzz/00000335.html. Accessed 14 January 2010.
Mikowychok, J.P. 2008. Controlling Invasives; Estimating the Costs of Native
Landscape Restoration. Pennsylvania Recreation and Park Society. Available
online at http://www.prps.org/pdf/08-5-12InvasivesControlCosts.pdf. Accessed
14 January 2010.
Miller, J.H. 2003. Nonnative Invasive Plants of Southern Forests: A Field Guide
for Identification and Control. Gen. Tech. Rep. SRS-62. USDA Forest Service,
Southern Research Station, Asheville, NC. 93 pp. Available online at http://www.
invasive.org/eastern/srs/. Accessed 14 January 2010.
Miller, J.H. 2005. Prevalent invasive trees. Available online at http://www.invasive.
org/weeds/ppt/treeshrubID.ppt. Accessed 14 January 2010.
Missouri Botanical Garden (MBG). 2009. PlantFinder Search. Available online
at http://www.mobot.org/gardeninghelp/plantfinder/plantkey.asp. Accessed 14
January 2010.
Mooney, H.A., and J.A. Drake. 1989. Biological invasions: A SCOPE program
overview. Pp. 491–506, In J.A. Drake, H.A. Mooney, F. di Castri, R.H. Groves,
F.J. Krueger, M. Rejmánek, and M. Williamson (Eds.). Biological Invasions:
A Global Perspective. Scientific Committee on Problems of the Environment
(SCOPE) of the International Council of Scientific Unions (ICSU). John Wiley
and Sons, New York, NY. 550 pp.
Moorhead, D.J. 2005. Invasive plant control in forests. Pp. 544–549, In P. Guillebeau
(Ed.). 2005 Georgia Pest Management Handbook. Special Bulletin 28. University
of Georgia, Athens, GA. Available online at http://www.ent.uga.edu/pmh/
Com_Trees.pdf. Accessed 14 January 2010.
Munger, G.T. 2003. Ligustrum spp. Fire Effects Information System. U.S. Department
of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences
Laboratory. Available online at http://www.fs.fed.us/database/feis/plants/
shrub/ligspp/introductory.html. Accessed 14 January 2010.
Muzika, R.-M., and J.M. Swearingen. 2009. PCA Alien Plant Working Group —
Russian-Olive (Elaeagnus angustifolia). Available online at http://www.nps.gov/
plants/alien/fact/elan1.htm. Accessed 14 January 2010.
2010 R.L. Boyce 23
Natural Lands Trust. 2007. Controlling Invasive Plants. Natural Lands Trust,
Media, PA. 4 pp. Available online at http://www.natlands.org/uploads/document_
619200892414.pdf. Accessed 14 January 2010.
Nature Conservancy Connecticut Chapter. 2009. The Nature Conservancy—Invasive
plant: Japanese Barberry. Available online at http://www.nature.org/wherewework/
northamerica/states/connecticut/science/art23895.html. Accessed 14 January
2010.
Ohio State University. 2010. Viburnum lantana. Available online at http://hcs.osu.
edu/hcs/TMI/Plantlist/vi_ntana.html. Accessed 14 January 2010.
Oliver, P.A., D.A. Orwin, M.J. Beauchamp, and D.J. Bullock. 2001. The potential
use of the goat (Capra hircus) for the restoration of scrub-invaded chalk grassland.
Land Contamination & Reclamation 9:225-231.
Paghat's Garden. 2005. Paghat's Garden: Spiraea japonica 'Bumalda'. Available online
at http://www.paghat.com/spiraeajaponica.html. Accessed 14 January 2010.
Pennsylvania Department of Conservation and Natural Resources (DCNR). 2010.
Invasive Exotic Plant Tutorial – viburnum MC.pdf. Available online at http://
www.dcnr.state.pa.us/forestry/invasivetutorial/viburnum_M_C.htm. Accessed
14 January 2010.
Plants for a Future (PFAF). 2008. PFAF Database Search. Available online at http://
www.pfaf.org/index.php. Accessed 14 January 2010.
Plant Information Databases. 2010. UF Environmental Plant Information Databases.
University of Florida/Institute of Food and Agricultural Sciences. Available on
line at http://hort.ifas.ufl.edu/database/index.shtml. Accessed 14 January 2010.
R Development Core Team. 2008. R: A language and environment for statistical
computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN
3-900051-07-0. Available online at http://www.R-project.org. Accessed 8 December
2008.
Randall, J.M., and J. Marinelli. 1996. Invasive Plants: Weeds of the Global Garden.
Brooklyn Botanic Garden, Brooklyn, NY. 112 pp.
Rehder, A. 1986. Manual of Cultivated Trees and Shrubs Hardy in North America:
Exclusive of the Subtropical and Warmer Regions. 2nd Edition, revised and enlarged.
Dioscorides Press, Portland, OR. 996 pp.
Rejmánek, M. 1989. Invasibility of plant communities. Pp. 369–388, In J.A. Drake,
H.A. Mooney, F. di Castri, R.H. Groves, F.J. Krueger, M. Rejmánek, and M.
Williamson (Eds.). Biological Invasions: A Global Perspective. Scientific Committee
on Problems of the Environment (SCOPE) of the International Council of
Scientific Unions (ICSU). John Wiley and Sons, New York, NY. 550 pp.
Remaley, T. 2005. PCA Alien Plant Working Group—Japanese Spiraea (Spiraea
japonica). Available online at http://www.nps.gov/plants/alien/fact/spja1.htm.
Accessed 8 December 2008.
Remaley, T. 2006. PCA Alien Plant Working Group—Silk Tree (Albizia julibrissin).
Available online at http://www.nps.gov/plants/alien/fact/alju1.htm. Accessed 8
December 2008.
Remaley, T. 2009a. PCA Alien Plant Working Group—Japanese Spiraea (Spiraea
japonica). Available online at http://www.nps.gov/plants/alien/fact/spja1.htm.
Accessed 14 January 2010.
Remaley, T. 2009b. PCA Alien Plant Working Group—Silk Tree (Albizia julibrissin).
Available online at http://www.nps.gov/plants/alien/fact/alju1.htm. Accessed 14
January 2010.
24 Northeastern Naturalist Vol. 17, Monograph No. 7
Rhoads, A.F., and T.A. Block. 2002. Invasive Plant Fact Sheet: Berberis thunbergii.
3 pp. Available online at http://www.paflora.org/Berberis%20thunbergii.pdf. Accessed
14 January 2010.
Rhodus, T. 2002. Plantfacts. Ohio State University. Available online at http://plantfacts.
osu.edu/. Accessed 14 January 2010.
Richardson, B. 2004. Plant Assessment Form. Crataegus monogyna. Available online
at http://www.cal-ipc.org/ip/inventory/PAF/Crataegus%20monogyna.pdf. 8
pp. Accessed 14 January 2010.
Roberts, D.W. 1986. Ordination on the basis of fuzzy set theory. Vegetatio
66:123–131.
Roberts, D.W. 2008. Statistical analysis of multidimensional fuzzy set ordinations.
Ecology 89:1246–1260.
Sather, N., and N. Eckardt. 1987. Element Stewardship Abstract for Elaeagnus
umbellata. 4 pp. Available online at http://www.imapinvasives.org/GIST/ESA/
esapages/documnts/elaeumb.pdf. Accessed 14 January 2010.
Smith, T.E. (Ed.). 1997. Missouri Vegetation Management Manual. Missouri Department
of Conservation, Jefferson City, MO. 161 pp. Available online at http://mdc.
mo.gov/nathis/exotic/vegman/. Accessed 14 January 2010.
South Australia Murray-Darling Basin Natural Resources Management Board (MDB
NRMB). 2007. Pest animal and plant control. Available online at http://www.
samdbnrm.sa.gov.au/BoardProjects/PestAnimalandPlantControl.aspx. Accessed
14 January 2010.
Southeast Exotic Pest Plant Council (SE-EPPC). 2003. Southeast Exotic Pest Plant
Council Invasive Plant Manual. Available online at http://www.invasive.org/
eastern/eppc/. Accessed 14 January 2010.
Southeast Regional Network of Expertise and Collection (SERNEC). 2010. SERNEC
States and Herbaria spreadsheet. Available online at http://www.sernec.
org/files/SERNEC-States%20and%20Herbaria_Final_1.xls. Accessed December
8, 2010.
Spencer, N. 2005. PCA Alien Plant Working Group—Wineberry (Rubus phoenicolasius).
Available online at http://www.nps.gov/plants/alien/fact/ruph1.htm. Accessed
8 December 2008.
Spencer, N. 2009. PCA Alien Plant Working Group – Wineberry (Rubus phoenicolasius).
Available online at http://www.nps.gov/plants/alien/fact/ruph1.htm. Accessed
14 January 2010.
Swearingen, J. 2009. PCA Alien Plant Working Group – Japanese Barberry (Berberis
thunbergii). Available online at http://www.nps.gov/plants/alien/fact/beth1.htm.
Accessed 14 January 2010.
Swearingen, J., K. Reshetiloff, B. Slattery, and S. Zwicker. 2002. Plant Invaders of
Mid-Atlantic Natural Areas. National Park Service and U.S. Fish and Wildlife
Service, Washington, DC. 82 pp. Available online at http://www.nps.gov/plants/
alien/pubs/midatlantic/. Accessed 14 January 2010.
Tasmania Department of Primary Industries and Water (DPIW). 2002. DPIW – Sweet
Briar – Control Guide. Available online at http://www.dpiw.tas.gov.au/inter.nsf/
WebPages/RPIO-4ZW385?open. Accessed 14 January 2010.
Tenaglia, D. 2007. Missouri flora web page. Available online at http://www.missouriplants.
com/. Accessed 14 January 2010.
The Ohio State University. 2010. Viburnum lantana. Available online at http://hcs.
osu.edu/hcs/TMI/Plantlist/vi_ntana.html. Accessed 14 January 2010.
2010 R.L. Boyce 25
Thomas, P.A., and A. Polwart. 2003. Taxus baccata L. Journal of Ecology 91:489-524.
Timberline Landscaping. 2010. Lonicera xylosteum L. and Lonicera x xylosteoides
Tausch.—Fly Honeysuckle, Emerald Mound Honeysuckle. 1 p. Available
online at http://www.timberlinelandscaping.com/Rev'dPlants-FRCC-TL/
Rev'dShrubsPDFs/LoniceraXxylosteoides-Fly,EmeraldMoundHoneysuckle.pdf.
Accessed 14 January 2010.
Tu, M. 2003. Element Stewardship Abstract for Elaeagnus angustifolia. 9 pp. Available
online at http://www.imapinvasives.org/GIST/ESA/esapages/documnts/
elaeang.pdf. Accessed 14 January 2010.
Tucker, G.C. 1996. Salix atrocinerea—an overlooked willow in New York State.
New York Flora Association Newsletter 7(2):2. Available online at http://www.
nyflora.org/Newsletters/NYFA%20Newsletter%20Vol%207_2%20(96).pdf. Accessed
14 January 2010.
Urbatsch, L. 2000. Exotic Weed Species. Chinese Privet. Ligustrum sinense. USDA
Natural Resources Conservation Service, Washington, DC. 5 pp. Available online
at http://plants.usda.gov/plantguide/pdf/pg_lisi.pdf. Accessed 14 January 2010.
US Census Bureau. 2005. Table 1: Annual Estimates of the Population for Counties
of Kentucky: April 1, 2000 to July 1, 2004 (CO-EST2004-01-21). Available
online at http://www.census.gov/popest/counties/tables/CO-EST2004-01-21.xls.
Accessed 14 January 2010.
USDA Natural Resources Conservation Service (NRCS). 2008. The PLANTS Database.
National Plant Data Center, Baton Rouge, LA 70874-4490 USA. Available
online at http://plants.usda.gov/. Accessed December 8, 2008.
Van Clef, M. 2008. Invasive Species Phenology and Treatment Recommendations.
Friends of Hopewell Valley Open Space, Pennington, NJ. 4 pp. Available online
at http://www.fohvos.org/pdfs/CJISSTPhenology_and_Treatment_Target_Species_
2008.pdf. Accessed 14 January 2010.
Victoria Department of Primary Industries (DPI). 2009. Invasiveness Assessment
– Purple Osier (Salix purpurea) in Victoria (Nox). Available online at http://
www.dpi.vic.gov.au/dpi/vro/vrosite.nsf/pages/invasive_purple_osier. Accessed
14 January 2010.
Washington State University (WSU) Clark County Extension. 2010. Leatherleaf
viburnum – Viburnum rhytidophyllum – PNW Plants. Available online at
http://www.pnwplants.wsu.edu/PlantDisplay.aspx?PlantID=129. Accessed 14
January 2010.
Weakley, A.S. 2008. Flora of the Carolinas, Virginia, and Georgia, and Surrounding
Areas. The University of North Carolina Herbarium, Chapel Hill, NC. 1015
pp. Available online at http://herbarium.unc.edu/flora.htm. Accessed 14 January
2010.
Webster, C.R., M.A. Jenkins, and S. Jose. 2006. Woody invaders and the challenges
they pose to forest ecosystems in the eastern United States. Journal of Forestry
104:366–374.
Weeds Australia. 2010. Weeds Australia—Weed identification. Available online at
http://www.weeds.org.au/cgi-bin/weedident.cgi?tpl=ibra.tpl&ibra=all. Accessed
14 January 2010.
Welch, W.C. 2001, Jan–Feb. Winter honeysuckle, Lonicera fragrantissima. Horticulture
Update (Texas Agr. Ext. Service). Available online at http://aggie-horticulture.
tamu.edu/extension/newsletters/hortupdate/jan01/art1jan.html. Accessed
14 January 2010.
26 Northeastern Naturalist Vol. 17, Monograph No. 7
Wieseler, S. 2009a. PCA Alien Plant Working Group—Common Buckthorn (Rhamnus
cathartica). Available online at http://www.nps.gov/plants/alien/fact/rhca1.
htm. Accessed 14 January 2010.
Wieseler, S. 2009b. PCA Alien Plant Working Group—Siberian Elm (Ulmus pumila).
Available online at http://www.nps.gov/plants/alien/fact/ulpu1.htm. Accessed 14
January 2010.
Williams, C.E. 2009. PCA Alien Plant Working Group – Exotic Bush Honeysuckles
(Lonicera spp.). Available online at http://www.nps.gov/plants/alien/fact/loni1.
htm. Accessed 14 January 2010.
Wilson, L., J. Davison, and E. Smith. 2006. Grazing and browsing guidelines for invasive
rangeland weeds. Pp. 142–167, In K. Launchbaugh (Ed.). Targeted Grazing:
A Natural Approach to Vegetation Management and Landscape Enhancement.
American Sheep Industry Association, Englewood, CO. 199 pp. Available online
at http://www.cnr.uidaho.edu/rx-grazing/Handbook/Chapter_15_Targeted_Grazing.
pdf. Accessed 14 January 2010.
Wisconsin Department of Natural Resources (WDNR). 2009. Invasive Species:
Plants. Available online at http://dnr.wi.gov/invasives/plants.asp. Accessed 14
January 2010.
Woods, K.D. 1993. Effects of invasion by Lonicera tatarica L. on herbs and tree seedlings
in four New England forests. American Midland Naturalist 130:62–74.
Zheng, H., Y. Wu, J. Ding, D. Binion, W. Fu, and R. Reardon. 2004. Invasive Plants
of Asian Origin Established in the United States and their Natural Enemies. Volume
1. USDA Forest Service FHTET-2004-05. 147 pp. Available online at http://
www.invasive.org/weeds/asian/. Accessed 14 January 2010.
2010 R.L. Boyce 27
Appendix 1. Invasive shrubs found in Kentucky. Data are taken from the PLANTS database (USDA NRCS 2008) and from the noted references. Botanical,
common, and family names follow the PLANTS database (USDA NRCS 2008). Native range and introduction date, if known, are also given. Some introduction
dates, especially those attributed to Rehder (1986), may refer to introductions to Europe. A species is noted as currently distributed if described as such in the
literature or found for sale on the internet by a US distributor. Note that almost all species are available internationally. Date = US introduction date, # = number
of counties in which species was found, and Current? = currently distributed in Kentucky. Numerical superscript references to sources: 1. Rehder 1986. 2. Browne
1846. 3. PFAF 2008. 4. FHP Invasive Plants 2010. 5. Miller 2003. 6. SE-EPPC 2003. 7. Remaley 2009b. 8. Mehrhoff et al. 2004. 9. Swearingen et al. 2002.
10. Rhoads and Block 2002. 11. WDNR 2009. 12. Bargeron et al. 2003. 13. Alverson and Sigg 2005. 14. Muzika and Swearingen 2009. 15. Deiter 2005. 16. Tu
2003. 17. Smith 1997. 18. Sather and Eckhart 1987. 19. Martin 2005. 20. Brand 2001. 21. Frappier et al. 2004. 22. Christman 2008. 23. Batcher 2000. 24. Zheng
et al. 2004. 25. Urbatsch 2000. 26. Langeland and Burks 1998. 27. Welch 2001. 28. Batcher and Stiles 2000. 29. Luken and Thieret 1995. 30. Gorchov and Trissel
2003. 31. Hutchinson and Vankat 1997. 32. Gould and Gorchov 2000. 33. Woods 1993. 34. Converse 1984. 35. Gooseberry Limited 2010. 36. Hedgerowmobile
2010. 37. Amrine 2002. 38. Eckardt 1987. 39. Hoshovsky 1989. 40. Hoshovsky 2005. 41. Johnson and Hoagland 1999. 42. Weakley 2008. 43. Spencer 2009.
44. GRIN 2009. 45. Tucker 1996. 46. Remaley 2009a. 47. Tenaglia 2007. 48. Adams 2004. 49. Cochran 2000. 50. Wieseler 2009b.
Species Common name Family Native range Date # Current?
Acer campestre L. Hedge Maple Aceraceae Europe, N Africa, W Asia1 18222 1 Yes
A. ginnala Maxim. Amur Maple Aceraceae N China, Korea, Japan1 ≈18601 1 Yes
Aesculus parviflora Walter Bottlebrush Buckeye Hippocastanaceae S Carolina, Alabama to Florida1 17851 1 Yes
Albizia julibrissin Durazz. Silktree Fabaceae S to E Asia3 17451, 3–7 46 Yes
Berberis thunbergii DC. Japanese Barberry Berberidaceae Japan1, 8–12 18641, 8–11 25 Yes
Callicarpa dichotoma (Lour.) Purple Beautyberry Verbenaceae China, Japan1 18571 1 Yes
K. Koch
Caryopteris x clandonensis Bluebeard Verbenaceae Hybrid between C. incana 19331 1 Yes
hort. ex Rehder and C. mongholica1
Chaenomeles speciosa Flowering Quince Rosaceae China1, 3 Before 18001 13 Yes
(Sweet) Nakai
Crataegus monogyna Jacq. Oneseed Hawthorn Rosaceae Europe, N Africa, S Asia3, 13 1800s13 2 Yes
Deutzia scabra Thunb. Fuzzy Pride-of-Rochester Hydrangeaceae Japan, China1, 3–4 18221 6 Yes
Elaeagnus angustifolia L. Russian Olive Elaeagnaceae Europe, W Asia1, 5, 14–16 Late 1800s14 10 No
E. pungens Thunb. Thorny Olive Elaeagnaceae Japan1 18301 1 Yes
E. umbellata Thunb. Autumn Olive Elaeagnaceae China, Korea, Japan1, 5–6, 9, 17–18 18301, 5, 9, 17–18 52 Yes
Eleutherococcus pentaphyllus Ginseng Araliaceae Japan1 18591 4 Yes
(Siebold & Zucc.) Nakai
28 Northeastern Naturalist Vol. 17, Monograph No. 7
Species Common name Family Native range Date # Current?
Euonymus alatus (Thunb.) Winged Burning Bush Celastraceae NE Asia, Japan, central ≈18605, 9, 19 46 Yes
Siebold China1, 3, 5, 9, 19
E. europaeus L. European Spindletree Elaeagnaceae Europe, W Asia1 ND 1 Yes
E. kiautschovicus Loes. Creeping Strawberry Bush Celastraceae China1, 20 ≈18491 1 Yes
Forsythia suspensa (Thun.) Weeping Forsythia Oleaceae China1 18331 2 Yes
Vahl
F. viridissima Lindl. Green-stemmed Forsythia Oleaceae China1 19171 1 Yes
Frangula alnus Mill. Glossy Buckthorn Rhamnaceae Europe, W Asia, N Africa1, 8, 21 Before 18001, 8 4 Yes
Hibiscus syriacus L. Rose of Sharon Malvaceae E Asia1, 3–4, 22 1600s?1, 22 18 Yes
Ilex cornuta Lindl. & Paxton Chinese Holly Aquifoliaceae E China1 18461 1 Yes
Kerria japonica (L.) DC. Japanese Rose Rosaceae China, Japan1 18341 4 Yes
Ligustrum amurense Carrière Amur Privet Oleaceae China1, 23 18601 5 Yes
L. obtusifolium Siebold & Border Privet Oleaceae Japan1, 8, 23 18858 17 Yes
Zucc.
L. ovalifolium Hassk. California Privet Oleaceae Japan1, 21 18471 4 Yes
L. sinense Lour. Chinese Privet Oleaceae China1, 5, 8, 23–26 18521, 25–26 40 Yes
L. vulgare L. European Privet Oleaceae Europe, N Africa1, 5, 23 Mid-1800s5 22 Yes
Lonicera fragrantissima Sweet Breath of Spring Caprifoliaceae China24, 27 After 18451, 27 3 Yes
Lindl. & Paxton
L. maackii (Rupr.) Amur Honeysuckle Caprifoliaceae China, Korea, Japan1, 5, 8, 24, 28–32 1890s8, 28–32 47 Yes
Herder
L. morrowii A. Gray Morrow’s Honeysuckle Caprifoliaceae Japan1, 6, 8, 28 ≈18751, 6, 8, 28 8 Yes
L. standishii Jacques Standish’s Honeysuckle Caprifoliaceae Europe, Asia1, 9 ≈18451 2 Yes
L. tatarica L. Tatarian Honeysuckle Caprifoliaceae C Asia, S Russia1, 6, 8, 28, 33 17521, 6, 8, 28, 33 4 Yes
L. xylosteum L. Dwarf Honeysuckle Caprifoliaceae Europe, Asia1, 8 Before 19611 3 Yes
Lonicera x bella Zabel Bell’s Honeysuckle Caprifoliaceae Hybrid between L. morrowii Before 18781, 4 1 Yes
and L. tatarica1, 4, 8, 28
Lonicera x minutiflora Zabel Small-flowered Honeysuckle Caprifoliaceae Hybrid between L. morrowii 18781 2 No
and L. xylosteoides1
Lonicera x xylosteoides Tausch Fly Honeysuckle Caprifoliaceae Hybrid between L. tatarica Before 18381 1 Yes
and L. xylosteum1
Malus baccata (L.) Borkh. Siberian Crab Apple Rosaceae E Asia1, 3 17841 2 Yes
2010 R.L. Boyce 29
Species Common name Family Native range Date # Current?
Morus alba L. White Mulberry Moraceae E Asia1, 9, 24 1700s9 48 Yes
Philadelphus coronarius L. Sweet Mock Orange Hydrangeaceae Europe1 Before 17701 2 Yes
Platycladus orientalis (L.) Oriental Arborvitae Cupressaceae N China, Korea1 Before 17371 1 Yes
Franco
Prunus cerasus L. Sour Cherry Rosaceae SE Europe to W Asia1, 3 ND 7 Yes
P. mahaleb L. Mahaleb Cherry Rosaceae Europe, W. Asia1 Before 18462 20 Yes
Pyracantha crenulata Nepalese Firethorn Rosaceae Himalayas1 ≈18441 1 Yes
(D. Don) Roem.
Rhamnus cathartica L. Common Buckthorn Rhamnaceae Europe, N Africa, N and Before 18008, 34 9 No
W Asia1, 3–4, 9, 34
R. davurica Pall. Dahurian Buckthorn Rhamnaceae E Asia1, 3 18171 7 Yes
Rhodotypos scandens Jetbead Rosaceae China, Korea, Japan1, 9 18661, 9 5 Yes
(Thunb.) Makino
Ribes uva-crispa L. European Gooseberry Grossulariaceae Europe, NW Africa, SW Asia3, 35 ND 1 Yes
Rosa canina L. Dog Rose Rosaceae Europe1, 3, 36 ND 10 Yes
R. eglanteria L. Sweetbriar Rose Rosaceae Europe1, 3 ND 21 Yes
R. micrantha Borrer ex Sm. Smallflower Sweetbrier Rosaceae Europe, Mediterranean1, 3 ND 3 No
R. multiflora Thunb. Rambler Rose Rosaceae Japan, Korea, China6, 8–9, 37–38 Before 18686, 8–9, 38 84 Yes
R. wichuraiana Crép. Memorial Rose Rosaceae E Asia1, 3 18911 17 Yes
Rubus armeniacus Focke Himalayan Blackberry Rosaceae W Europe1, 39–40 18851, 39–40 2 No
R. bifrons Vest ex Tratt. Himalayan Berry Rosaceae Europe3, 41–42 ND 2 No
R. phoenicolasius Maxim. Wineberry Rosaceae Japan, Korea, China1, 8–9, 43 18761, 8–9, 43 18 Yes
Salix atrocinerea Brot.A Large Gray Willow Salicaceae Europe, N Africa, Asia1, 3, 44–45 ND 5 No
S. purpurea L. Purpleosier Willow Salicaceae Europe, central Asia, N Africa, ND 2 Yes
Japan1, 3, 20
Spiraea japonica L. f. Japanese Meadowsweet Rosaceae Japan, Korea, China1, 3–4, 6, 46 18706, 8, 46 12 Yes
S. prunifolia Siebold & Zucc. Bridalwreath Spirea Rosaceae E Asia1, 3, 20, 47 18641 3 Yes
Syringa vulgaris L. Common Lilac Oleaceae SE Europe42, 44 1700s1, 48 7 Yes
Taxus baccata L. English Yew Taxaceae Europe, N Africa, W Asia1 early 1900s49 1 Yes
Ulmus pumila L. Siberian Elm Ulmaceae N China, E Siberia, Manchuria, 18601 13 Yes
Korea1, 3, 50
30 Northeastern Naturalist Vol. 17, Monograph No. 7
Species Common name Family Native range Date # Current?
Viburnum lantana L. Wayfaringtree Caprifoliaceae Europe, W. Asia1 ND 1 Yes
V. opulus L. var. opulus European Cranberrybush Caprifoliaceae Europe, N Africa, N Asia1, 3–4, 44 After 16501 8 No
V. rhytidophyllum Hemsl. Leatherleaf Arrowwood Caprifoliaceae C and W China1 19001 1 Yes
V. setigerum Hance Tea Viburnum Caprifoliaceae China1 19011 2 Yes
V. sieboldii Miq. Siebold’s Arrowwood Caprifoliaceae Japan1 18801 1 Yes
AThe USDA PLANTS database (USDA NRCS 2008) classifies Salix cinerea L. ssp. oleifolia (Sm.) Macreight as a synonym for S. atrocinerea and S. cinerea L. ssp.
cinerea as a synonym for S. cinerea L. Clark and Weckman (2008) classified both as subspecies of S. cinerea and mapped them together, so some of the listed county
occurrences may not be S. atrocinerea.
2010 R.L. Boyce 31
Appendix 2. Physiological characteristics and soil and moisture requirements of invasive shrubs. For shade tolerance, T = totally shade tolerant, P = partially
tolerant, and I = intolerant. For leaf habit, E = evergreen, DE = deciduous-extended, and D = deciduous. Under soils requirements, WR = wide range. Nitrogenfixing species are also noted. ND = no data were found. Numerical superscript source references: 1. PFAF 2008. 2. FHP Invasive Plants 2010. 3. Remaley 2009b.
4. Miller 2003. 5. SE-EPPC 2003. 6. Swearingen 2009. 7. Swearingen et al. 2002. 8. Rhoads and Block 2002. 9. Randall and Marinelli 1996. 10. Mehrhoff et
al. 2004. 11. MBG 2009. 12. Evans 2003. 13. Brand 2001. 14. Alverson and Sigg 2005. 15. Muzika and Swearingen 2009. 16. Brusati 2005. 17. Deiter 2005.
18. Tu 2003. 19. Sather and Eckardt 1987. 20. Martin 2005. 21. BackyardGardener 2010. 22. Converse 1984. 23. Plant Information Databases 2010. 24. Faucon
2005. 25. Bushes and Shrubs 2003. 26. Horticopia 2004. 27. Urbatsch 2000. 28. Williams 2009. 29. Zheng et al. 2004. 30. Welch 2001. 31. Batcher and Stiles
2000. 32. Boiteau et al. 2009. 33. Woods 1993. 34. Kling 2010. 35. Timberline Landscaping 2010. 36. Wieseler 2009a. 37. Hoch 2001. 38. Albrecht 2001. 39.
Bergmann and Swearingen 2009. 40. Eckardt 1987. 41. Dana and Lerner 2006. 42. Langeland and Burks 1998. 43. Hoshovsky 1989. 44. Hoshovsky 2005. 45.
Spencer 2009. 46. Rhodus 2002. 47. Remaley 2009a. 48. Paghat’s Garden 2005. 49. Wieseler 2009b. 50. Invasive Species 2009. 51. Kline 2010. 52. WSU Clark
County Extension 2010.
Shade
Species tolerance Leaf habit Soil requirements Moisture requirements N-fixer
Acer campestre P1 D1 WR, tolerates calcareous1 Moist1 No
Acer ginnala P1 D1 WR1 Moist1 No
Aesculus parviflora T1 D1 WR1 Moist well-drained1 No
Albizia julibrissin P1–5 D1–2, 4 WR1–3 WR1–2, 4 Yes1–2, 4
Berberis thunbergii T5–9 E or D5–8, 9–10 WR1, 9 Drought-tolerant1, 5–6 No
Callicarpa dichotoma P11-12 D11-12 WR12 WR12 No
Caryopteris x clandonensis I11, 13 D11, 13 Average11 Drought-tolerant11 No
Chaenomeles speciosa T1 D1 WR, not high pH1 Drought-tolerant11 No
Crataegus monogyna P1, 9 D1, 9, 14 WR1, 14 Drought-tolerant1 No
Deutzia scabra P1 D1–2 WR1 WR2 No
Elaeagnus angustifolia P15–16 D4, 9, 17 WR, pH 6–99–10, 15–18 Dry to moist9–10, 15–18 Yes4, 10
E. pungens T1, 4 E1, 4 WR, tolerates salt1, 4 Drought-tolerant1, 4 Yes1
E. umbellata I19 DE4, 9, 19 Tolerates poor soils, low pH10, 19 Drought-tolerant4–5, 7, 10, 19 Yes4–5, 7, 10, 19
Eleutherococcus pentaphyllus P1, 11, 13 DE13 WR1, 11, 13 Drought-tolerant11, 13 No
Euonymus alatus T1, 9–10, 20 D1, 4, 9–10, 20 WR1, 10, 20 Well-drained, dry to moist1, 9–10 No
E. europaeus P1, 13 DE13 WR1, 13 WR but well-drained1, 13 No
E. kiautschovicus P13, 21 DE13, 21 WR13, 21 Well-drained13, 21 No
Forsythia suspensa T1, 11 D1, 11 WR1 Moist well-drained1 No
F. viridissima P1, 11, 13 D1, 11, 13 WR1, 11, 13 Moist well-drained1, 11 No
32 Northeastern Naturalist Vol. 17, Monograph No. 7
Shade
Species tolerance Leaf habit Soil requirements Moisture requirements N-fixer
Frangula alnus I10, 22 DE22 WR22 Tolerates high moisture2, 22 No
Hibiscus syriacus P1, 23 D1–2, 23 WR1, 23 Well-drained1–2, 23 No
Ilex cornuta P1, 11 E1, 11 WR1, 11 Moist well-drained1, 11 No
Kerria japonica T1, 11, 13 D1, 11, 13 WR1 Moist well-drained1, 11, 13 No
Ligustrum amurense P24 DE24 WR24 Well-drained24 No
L. obtusifolium P1, 26 E, DE1, 10 WR1 Moist well-drained1, 26 No
L. ovalifolium T1 E1 WR1 WR1 No
L. sinense T4, 27 E, DE1, 4, 9, 27 WR1, 26 Mesic best1, 26 No
L. vulgare P1 DE27 WR1 WR1 No
Lonicera fragrantissima P4, 28 DE4, 28–30 WR, including acidic and poor30 Drought-tolerant30 No
L. maackii P4, 10, 28, 31 DE1, 4, 9–10, 28, 30 WR, prefers calcareous10, 31 WR13 No
L. morrowii P4, 28, 31 DE4–5, 32 WR, prefers calcareous10, 31 Mesic best10, 31 No
L. standishii P28 DE, D28, 32 WR28 Mesic best28 No
L. tatarica P4–5, 10, 28 DE4–5, 28–29, 31, 33 WR31 WR31 No
L. xylosteum P10, 28 D28 WR10 WR10 No
Lonicera x bella P2, 10, 31 DE5, 28 WR31 WR31 No
Lonicera x minutiflora ND ND ND ND No
Lonicera x xylosteoides P34–35 DE34 WR34–35 Moist well-drained35 No
Malus baccata P1 D1 WR1 Moist well-drained1 No
Morus alba P1 D1 WR1 Drought-tolerant1 No
Philadelphus coronarius P13 D13 WR13 WR34 No
Platycladus orientalis P11. 13. 23 E11. 13. 23 WR13, 23 WR13, 23 No
Prunus cerasus P1 D1 WR1 Moist well-drained1 No
P. mahaleb P1 D1 WR1 Moist well-drained1 No
Pyracantha crenulata P1 E1 WR1 Moist well-drained1 No
Rhamnus cathartica P1–2, 10, 22 DE10 WR, neutral-basic pH, WR1–2, 10, 36 No
prefers calcareous1–2, 10, 22, 36
Rhamnus davurica P1 D1 WR1 Moist1 No
Rhodotypos scandens T2, 11, 37–38 D2, 7, 11, 37–38 WR2, 11, 37–38 WR11, 37–38 No
Ribes uva-crispa P1 D1 WR1 Moist well-drained1 No
Rosa canina P1 D1 WR1 Moist or wet, well-drained1 No
2010 R.L. Boyce 33
Shade
Species tolerance Leaf habit Soil requirements Moisture requirements N-fixer
R. eglanteria P1 D1, 11 WR1 Moist well-drained1, 11 No
R. micrantha P1 D1 WR, including high pH1 WR1 No
R. multiflora T5, 7, 39–40 D15 WR, prefers deep, fertile soils5, 7, 39–40 WR, prefers well-drained and No
moist5, 7, 39–40
R. wichuraiana P1 DE1, 41 WR1 Moist well-drained1 No
Rubus armeniacus I42 DE43–44 WR43–45 Moist, tolerates flooding43–44 No
R. bifrons P1 D1 WR1 Moist well-drained1 No
R. phoenicolasius I1, 10, 45 D10, 42 WR1 Moist well-drained42, 45 No
Salix atrocinerea I1 D1 WR1 Moist or wet1 No
S. purpurea I1, 34 D1, 13 WR1, 46 WR1, 13, 34, 46 No
Spiraea japonica P5, 47 D, DE2, 5, 47–48 WR2, 5, 7, 47 WR2, 5, 7, 47 No
S. prunifolia P1, 13 D1, 13 WR1, 49 Moist, well-drained1, 13 No
Syringa vulgaris I1 D1 WR, not acid1 Moist soil1 No
Taxus baccata T1, 13 E1, 11, 13 WR1, 13 Moist well-drained 1, 11, 13 No
Ulmus pumila P1 D1 WR1–2 WR1–2, 49–50 No
Viburnum lantana P1, 11, 13 D1, 11, 13 WR1, 13 WR1, 11, 13 No
V. opulus var. opulus P1 D1–2, 9, 51 WR, including high pH1–2, 9, 51 WR1–2, 9, 51 No
V. rhytidophyllum T13 E13 WR52 Well-drained13 No
V. setigerum P1, 11, 13 D1, 11, 13 WR1 Moist well-drained1, 11, 13 No
V. sieboldii P1, 13 D1, 13 WR1, 13 Moist well-drained1, 13 No
34 Northeastern Naturalist Vol. 17, Monograph No. 7
Appendix 3. Control methods available for invasive shrubs. Documentation of resprouting after cutting is listed in footnotes after species names, as this affects
efficiency of control methods. For biological control, NK = none known; this was used if references explicitly stated that no biological control methods
were known. ND = no data were found. Numerical superscript source references: 1. ISSG 2010. 2. FHP Invasive Plants 2010. 3. Plant Information Databases
2010. 4. PFAF 2008. 5. Remaley 2009b. 6. SE-EPPC 2003. 7. Miller 2003. 8. Randall and Marinelli 1996. 9. Nature Conservancy Connecticut Chapter 2009.
10. Swearingen 2009. 11. Swearingen et al. 2002. 12. WDNR 2009. 31. Rhoads and Block 2002. 14. MSU Extension 1999. 15. MBG 2009. 16. Alverson and
Sigg 2005. 17. Weeds Australia 2010. 18. Richardson 2004. 19. Holst and Simmonds 2001. 20. ABC 2008. 21. Tu 2003. 20. Brusati 2005. 23. Deiter 2005.
24. Moorhead 2005. 25. IPAMS 2010. 26. Mikowychok 2008. 27. Sather and Eckardt 1987. 28. Van Clef 2008. 29. Martin 2005. 30. Converse 1984. 31. Batcher
2000. 32. Ensbey 2004. 33. Munger 2003. 34. Langeland and Stocker 2001. 35. Williams 2009. 36. Smith 1997. 37. Batcher and Stiles 2000. 38. Center for
Earth and Environmental Science 2005. 39. Wieseler 2009a. 40. Mehrhoff et al. 2004. 41. Heidorn 1990. 42. South Australia MDB NRMB 2007. 43. Tasmania
DPIW 2002. 44. Eckardt 1987. 45. Amrine 2002. 46. Bergmann and Swearingen 2009. 47. Hoshovsky 1989. 48. Spencer 2009. 49. King County Noxious Weed
Control Program 2005. 50. Wilson et al. 2006. 51. Biosecurity New Zealand 2008. 52. Anderson 2007. 53. Victoria DPI 2009. 54. Brand 2001. 55. Remaley
2009a. 56. CBCN 2010. 57. Thomas and Polwart 2003. 58. Wieseler 2009b. 59. Ohio State University 2010. 60. Oliver et al. 2001. 61. Natural Lands Trust 2007.
62. Pennsylvania DCNR 2010. 63. Cheltenham Township 2009.
Species Mechanical control Fire control Chemical control Biological control
Acer campestre ND ND ND ND
A. ginnala1 Hand pulling, cutting2 Partially effective1 Yes2 ND
Aesculus parviflora3 ND ND ND ND
Albizia julibrissin2, 4–6 Repeated cutting, girdling, uprooting2, 4–6 ND Yes2, 5–7 Possible6
Berberis thunbergii8–10 Uprooting6, 8–13 Perhaps8, 12 Yes6, 8–13 NK13
Callicarpa dichotoma ND ND ND ND
Caryopteris x clandonensis ND ND ND ND
Chaenomeles speciosa14–15 ND ND ND ND
Crataegus monogyna16–18 Pull seedlings16 ND Yes16 Some goat grazing19
Deutzia scabra20 Pull seedlings2 ND Yes2 ND
Elaeagnus angustifolia8, 21–23 Pull seedlings8, 21, 23 With other methods, Yes7–8, 21, 23–24 NK21, 23
stump burning8, 21, 23
E. pungens25 Hand pull26 Yes7 Yes25–26 NK25
E. umbellata27 Pull seedlings6, 8, 11, 27 Not effective8, 27 Yes7–8, 11, 27 ND
Eleutherococcus pentaphyllus Pulling, mowing28 ND Yes28 ND
Euonymus alatus11 Pull seedlings11, 29 ND Yes7–8, 11, 24, 29 ND
E. europaeus Hand pull26 ND Yes26 ND
E. kiautschovicus ND ND ND ND
2010 R.L. Boyce 35
Species Mechanical control Fire control Chemical control Biological control
Forsythia suspensa ND ND ND ND
F. viridissima ND ND ND ND
Frangula alnus2, 8, 30 Repeated cutting, girdling, uprooting2, 30 Usually not effective8, 30 Yes2, 8, 30 Perhaps2
Hibiscus syriacus Uprooting effective2 ND Yes2 ND
Ilex cornuta ND ND ND ND
Kerria japonica4 Cutting15 ND ND ND
Ligustrum amurense31 Uprooting6, 31–32 Perhaps33 Yes6, 31–32 NK6, 31
L. obtusifolium11, 31 Uprooting6, 11, 31–32 ND Yes6, 11, 31–32 NK6, 31
L. ovalifolium31 Uprooting11, 31–32 ND Yes8, 31–32 NK6, 31
L. sinense8, 11, 31 Uprooting6, 8, 11, 31–32 Partially effective31 Yes6–8, 11, 24, 34–36 Goat grazing19
Ligustrum vulgare8, 11, 34 Uprooting6, 8, 11, 34–35 Partially effective34 Yes6–8, 11, 24, 31–32, 34 NK6, 31
Lonicera fragrantissima35 Repeated clipping, uprooting11, 35 Perhaps35 Yes11, 29, 35 NK11, 35
L. maackii6, 8, 37 Repeated clipping, uprooting6, 8, 11, 35, 37 Not effective alone8, 35–37 Yes6, 8, 11, 24, 35–37 Perhaps36
L. morrowii6, 8, 37 Repeated clipping, uprooting6, 8, 11, 35, 37 Not effective alone8, 35–37 Yes6, 8, 11, 24, 35, 37 Perhaps36
L. standishii35 Repeated clipping, uprooting1, 35 Perhaps35 Yes11, 35 NK11, 35
L. tatarica6, 8, 37 Repeated clipping, uprooting6, 8, 11, 31, 37 Not effective alone8, 36–37 Yes6, 8, 11, 36–37 NK10, 36–37
L. xylosteum35 Repeated clipping, uprooting7, 35 Perhaps35 Yes7, 35 NK7, 35
Lonicera x bella6, 37 Repeated clipping, uprooting2, 6, 11, 35, 37 Not effective alone35, 37 Yes2, 6, 11, 35, 37 NK11, 35, 37
Lonicera x minutiflora ND ND ND ND
Lonicera x xylosteoides ND ND ND ND
Malus baccata ND ND ND ND
Morus alba38 Uprooting11 ND Yes11 ND
Philadelphus coronarius ND ND ND ND
Platycladus orientalis ND ND ND ND
Prunus cerasus4 ND ND ND ND
P. mahaleb ND ND ND ND
Pyracantha crenulata ND ND ND ND
Rhamnus cathartica8, 36, 39 Pull seedlings, repeated cutting8, 36, 39–40 Repeated burning8, 36, 39–40 Yes8, 36, 39–40 Perhaps2
R. davurica41 Repeated cutting, girdling41 Repeated burning41 Yes41 NK41
Rhodotypos scandens11 Uprooting2, 11 ND Yes32 ND
Ribes uva-crispa ND ND ND ND
Rosa canina4 Pull seedlings, plowing42 ND Yes42 Goat grazing19, 42
36 Northeastern Naturalist Vol. 17, Monograph No. 7
Species Mechanical control Fire control Chemical control Biological control
R. eglanteria4, 43 Pull seedlings, plowing42–43 ND Yes42–43 Goat, sheep
grazing19, 42–43
R. micrantha4 ND ND ND ND
R. multiflora6–7, 44 Repeated mowing, uprooting6, 8, 10, 32, 44 Perhaps44 Yes6–8, 10, 24, 44 Possible6, 10, 44–46
R. wichuraiana4 ND ND ND ND
Rubus armeniacus47–49 Uprooting, repeated cutting47–49 Not effective alone47–49 Yes47–49 Goat, sheep,
chicken grazing47–49
R. bifrons4 ND ND ND Goat, sheep grazing50
R. phoenicolasius11, 49 Uprooting11, 48 ND Yes11 ND
Salix atrocinerea51–52 ND ND ND ND
S. purpurea17, 53–54 ND ND ND ND
Spiraea japonica10, 55 Repeated cutting2, 10, 55 ND Yes2, 10, 55 ND
S. prunifolia54 ND ND ND ND
Syringa vulgaris11 Girdle56 ND Yes56 ND
Taxus baccata57 ND ND ND ND
Ulmus pumila4, 8 Pulled seedlings, shallow girdling8, 58 Regular burning8, 58 Yes8, 58 ND
Viburnum lantana59 Hand pull26 ND Yes26 Goat grazing60
V. opulus var. opulus4, 8 Pull seedlings8 ND Yes2, 8 ND
V. rhytidophyllum ND ND ND ND
V. setigerum ND ND ND ND
V. sieboldii Physical removal, mowing28, 61-62 ND Yes61-63 ND
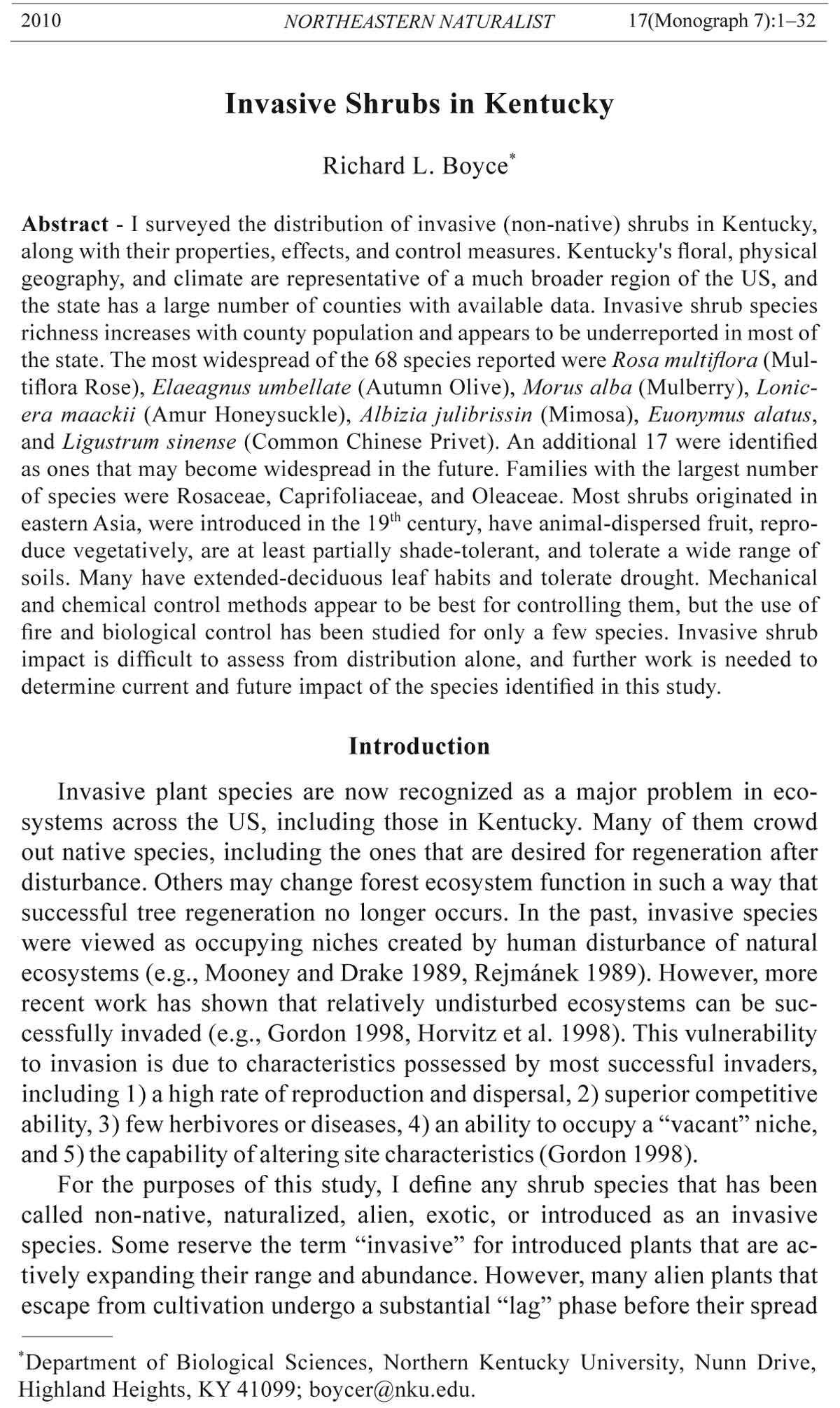













 The Northeastern Naturalist is a peer-reviewed journal that covers all aspects of natural history within northeastern North America. We welcome research articles, summary review papers, and observational notes.
The Northeastern Naturalist is a peer-reviewed journal that covers all aspects of natural history within northeastern North America. We welcome research articles, summary review papers, and observational notes.